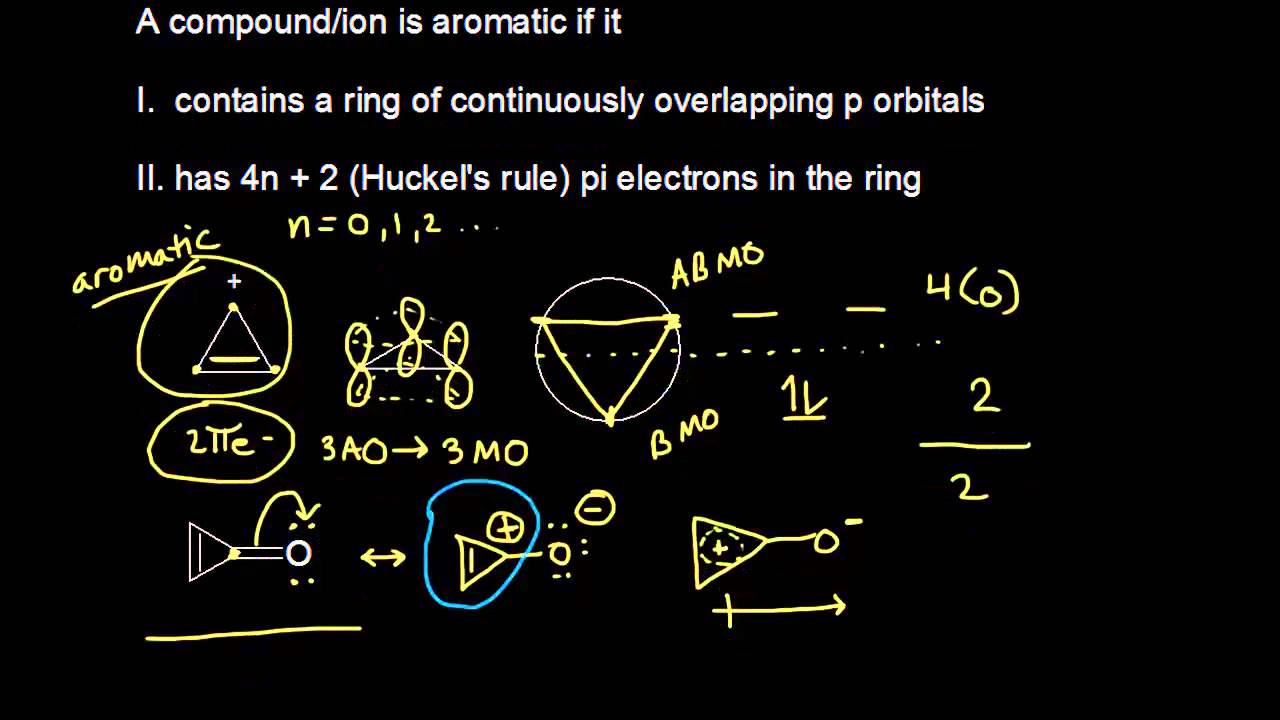
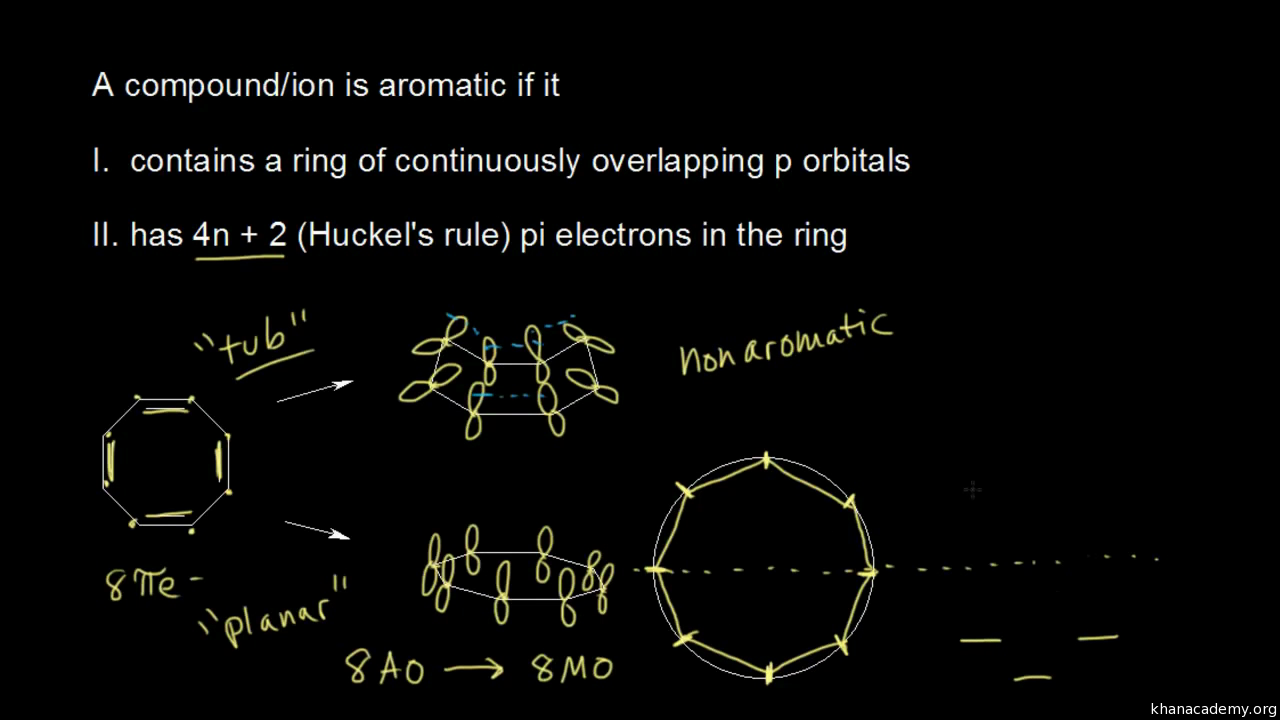
Aromatic Stability Iii Video Khan Academy
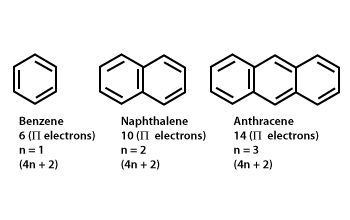
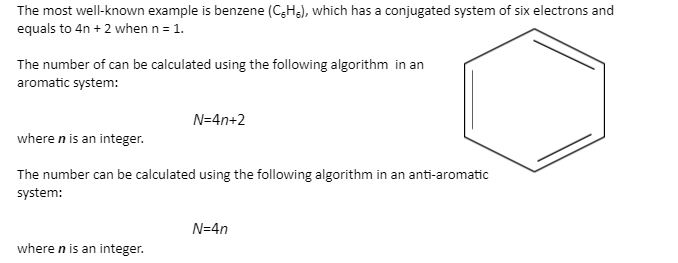
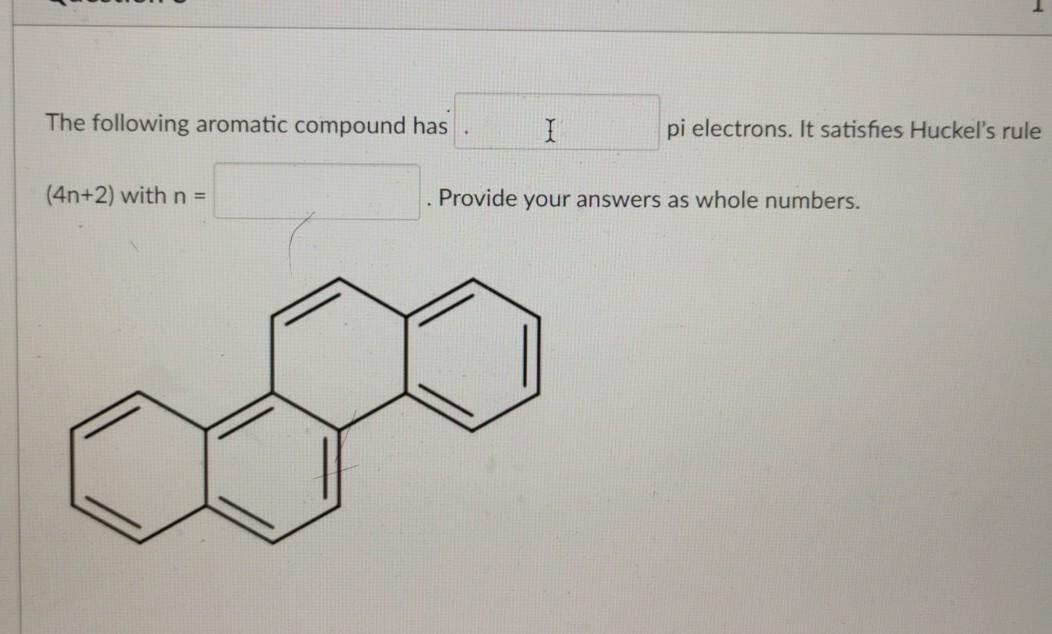
So, we find that that the number of electrons in the pi bonds are 3×2 (because there are two electrons in every double bond), so we see that the 6 electrons in the pi bonds, satisfiesTo apply the 4n2 rule, first count the number of π electrons in the molecule Then, set this number equal to 4n2 and solve for n If is 0 or any positive integer (1, 2, 3,), the rule has been
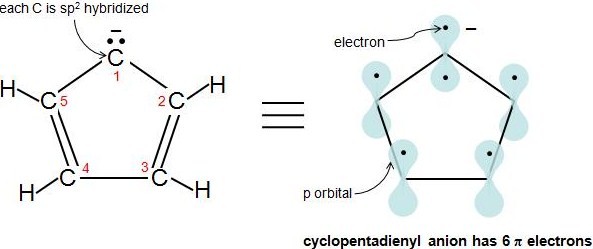
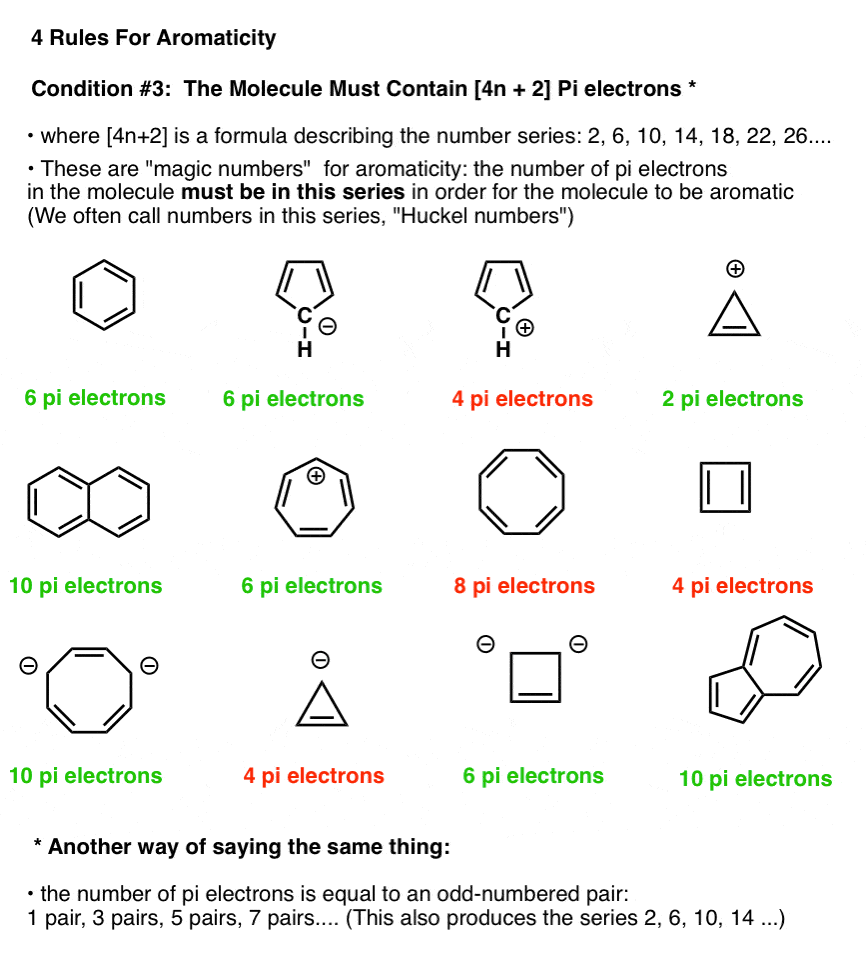
4n + 2 pi electron rule
4n + 2 pi electron rule-To join our iitjam/csirnet/iitgate/du/bhu/rpsc online regular/crash course please download the app and get registerd thereapp linkhttp//bitly/3b3zazIi) For the π cloud to be uninterrupted, every atom in the ring must have a p orbital iii) For the π cloud to be formed, each porbital must be able to overlap with the p− orbitals on either side of
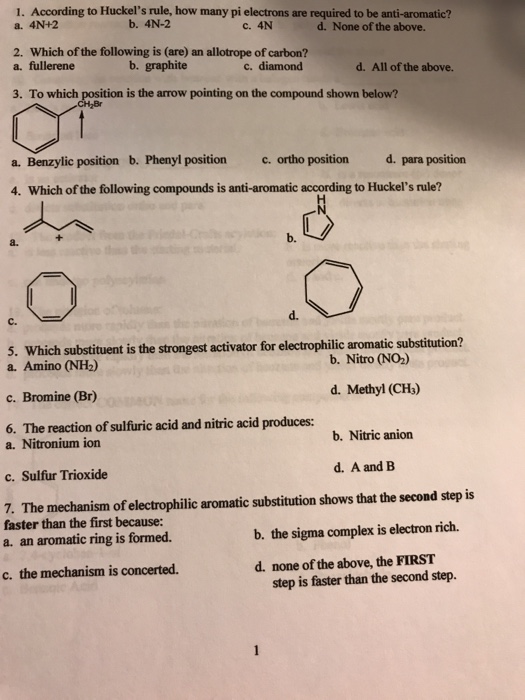
Solved 1 According To Huckel S Rule How Many Pi Electrons Chegg Com
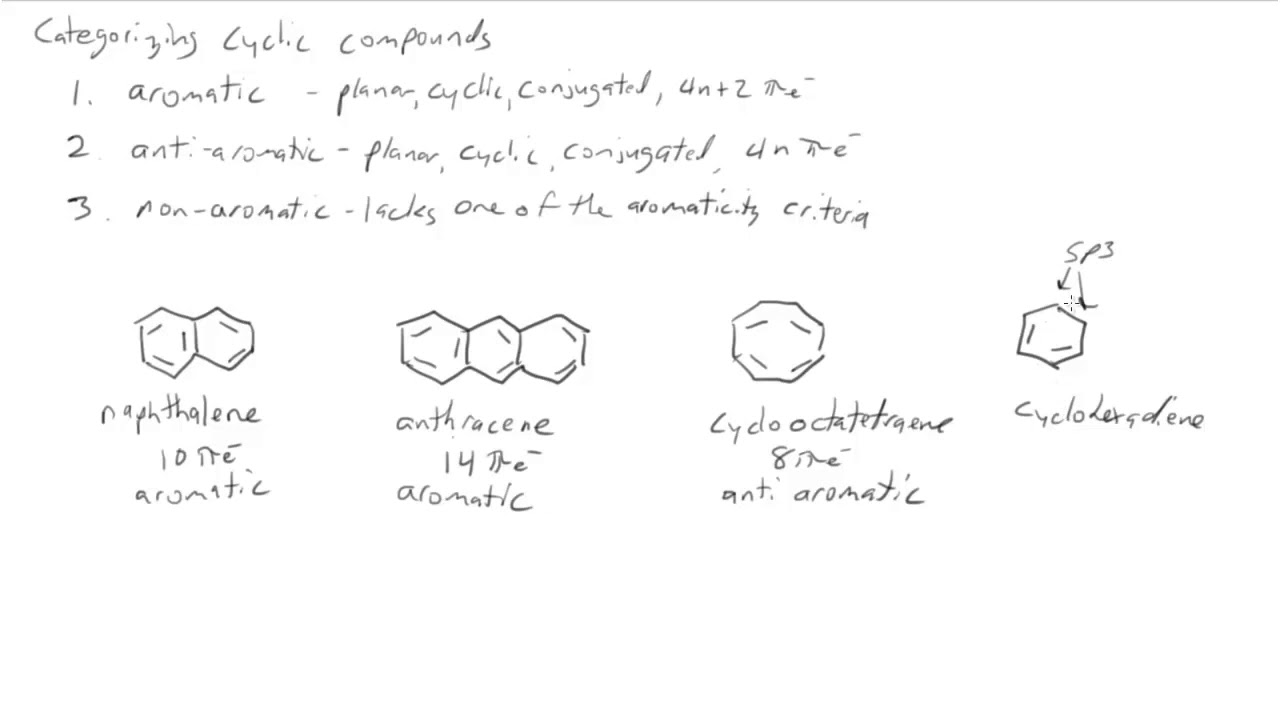
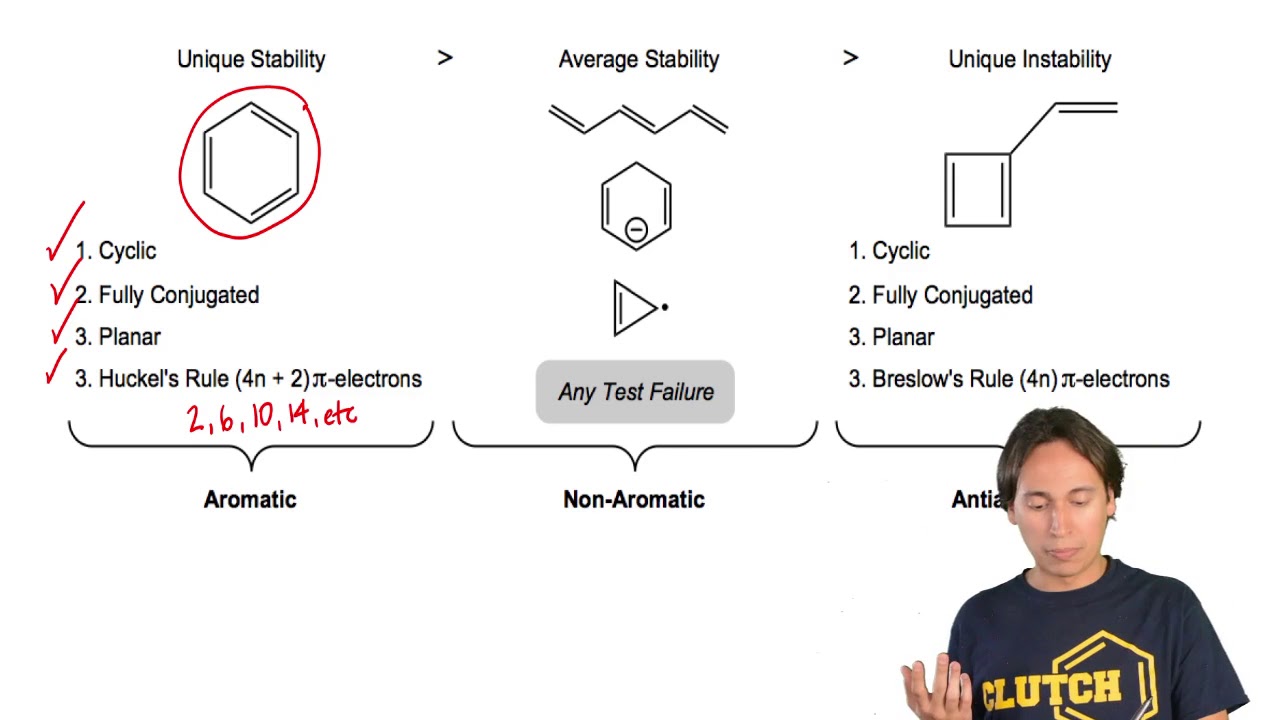
The Organic Chemistry Tutor This organic chemistry video tutorial shows you how to tell if a compound is aromatic, antiaromatic or nonaromatic by using huckel's rule / number of 4n2 piFor some reason none of my organic chemistry textbooks, lectures, etc explain what N actually is, so I made this videoHuckle's rule It states that if a cyclic, planar molecule has (4n2) pi electrons, it is considered aromatic
What is 4n 2 pi rule?How can we find 4n2= pi electron rule in huckles rule hamnahanif92 is waiting for your help Add your answer and earn pointsClick here👆to get an answer to your question ️ Huckel's Rule (4n 2) i electrons
4n + 2 pi electron ruleのギャラリー
各画像をクリックすると、ダウンロードまたは拡大表示できます
What Are Aromatic Compounds Socratic | 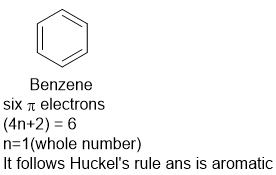 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
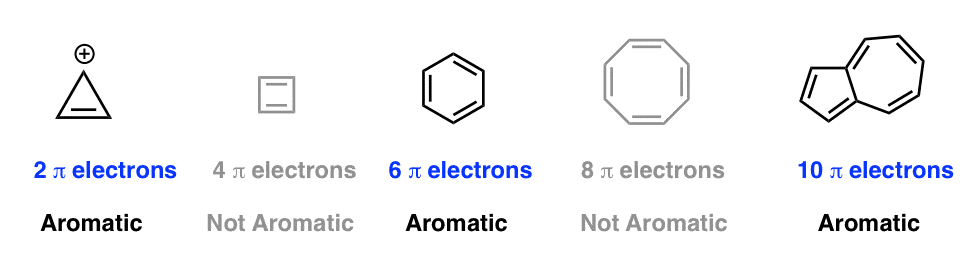 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
What Are Aromatic Compounds Socratic | 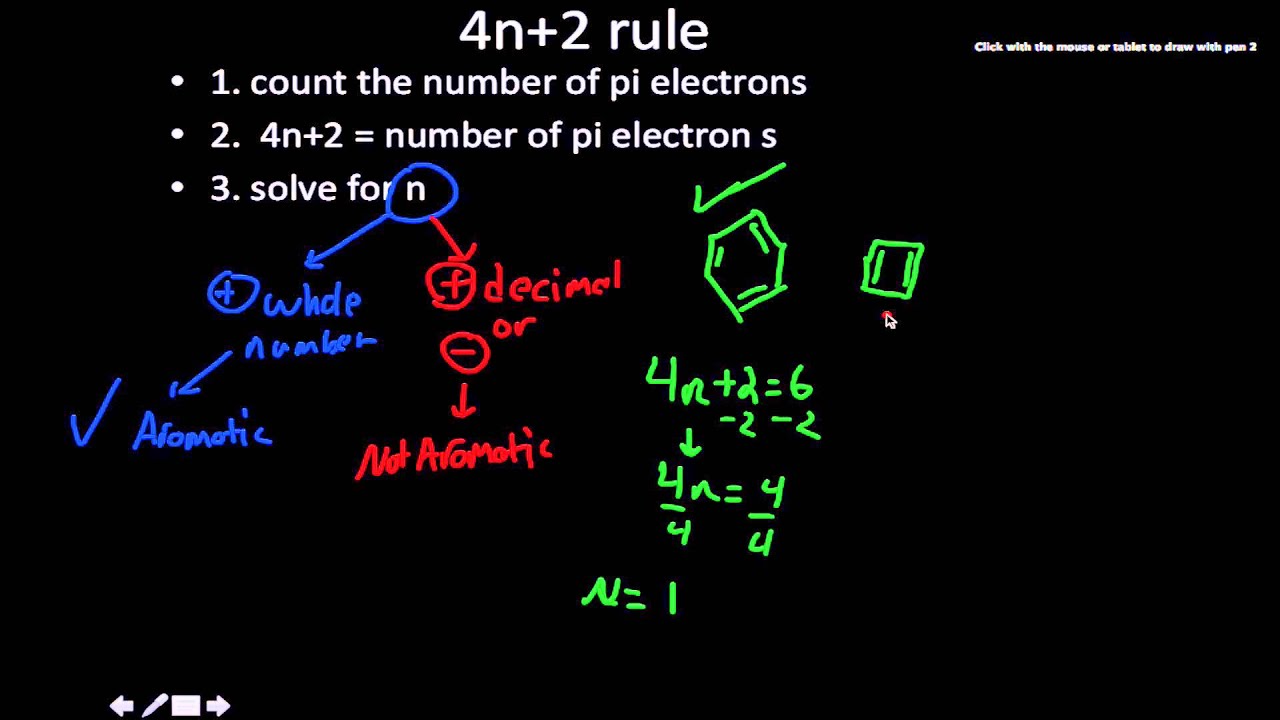 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
 What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic | 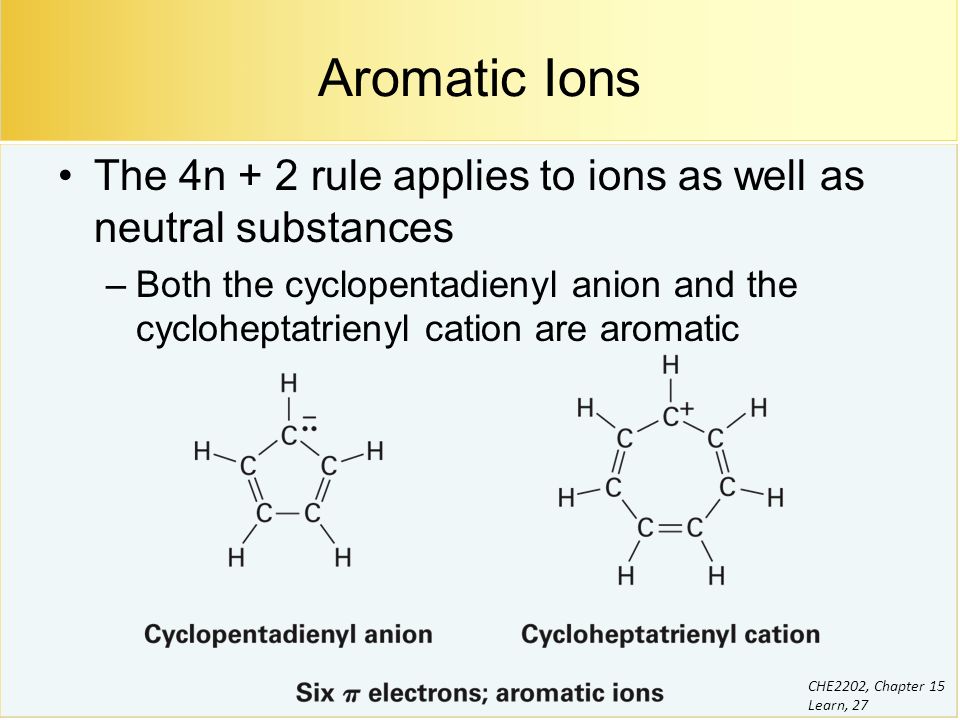 What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic | 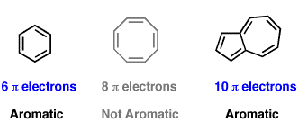 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
What Are Aromatic Compounds Socratic | 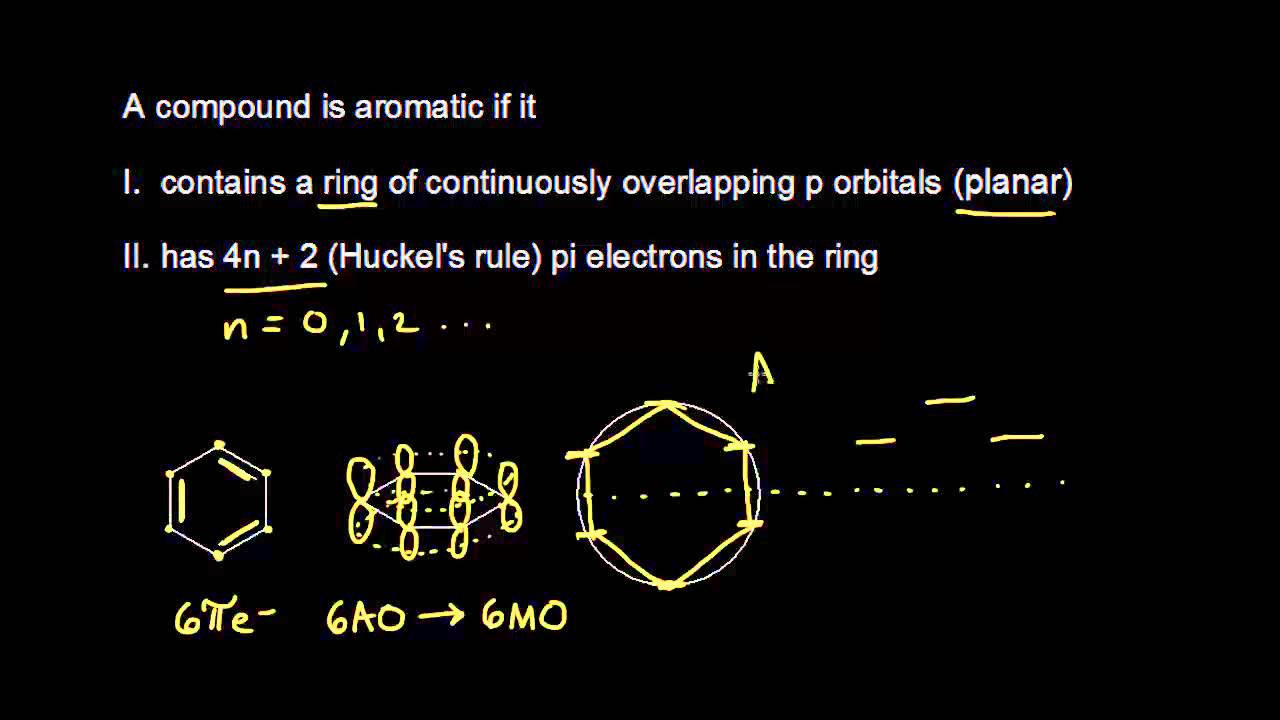 What Are Aromatic Compounds Socratic | 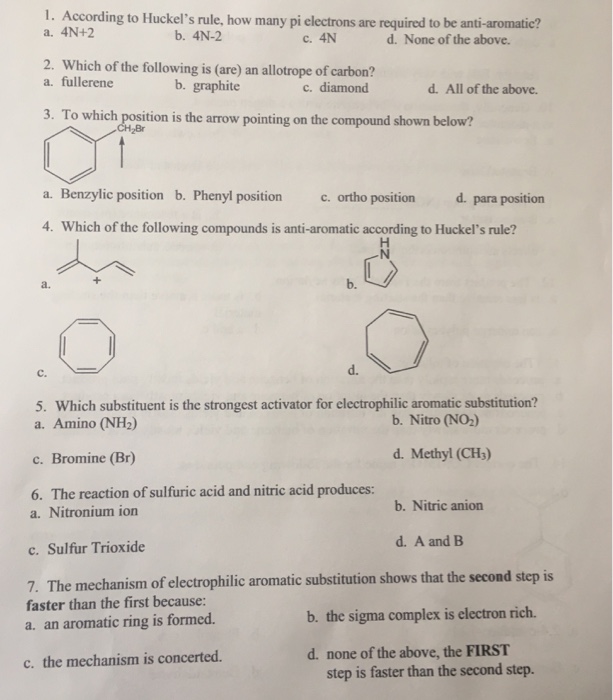 What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic | 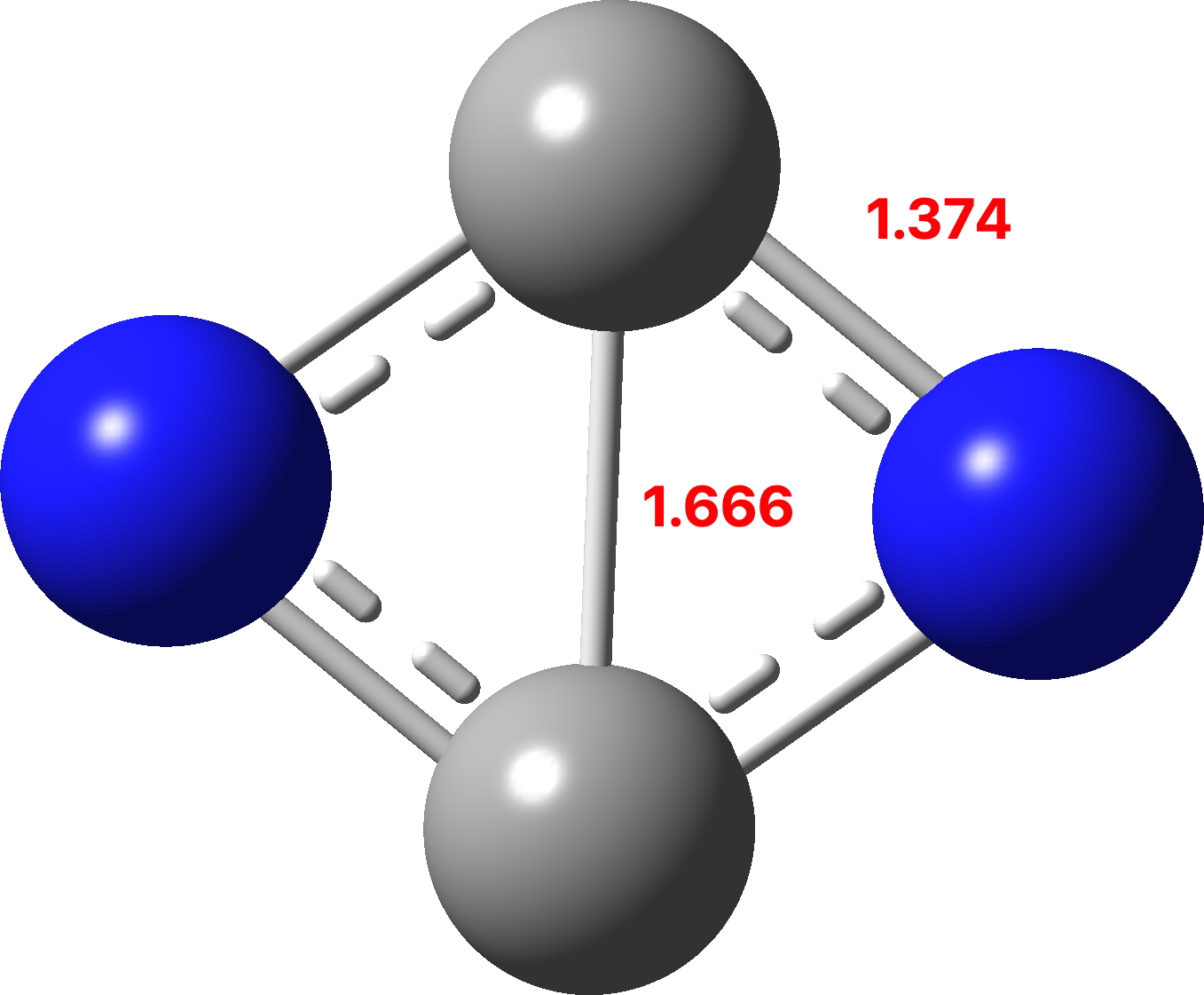 What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic | 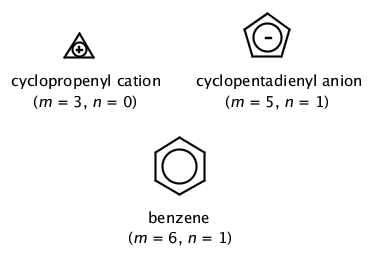 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic | 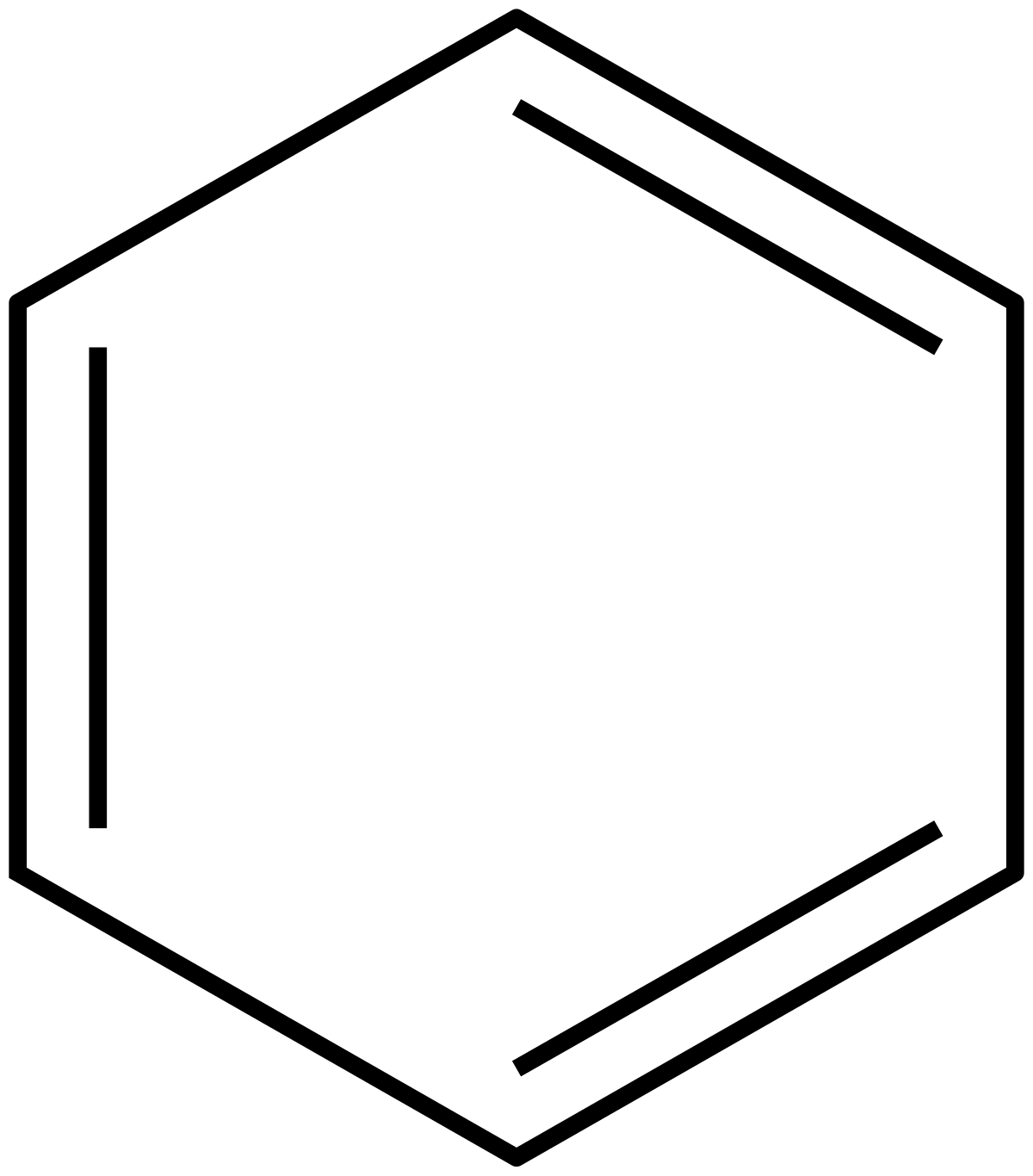 What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic | 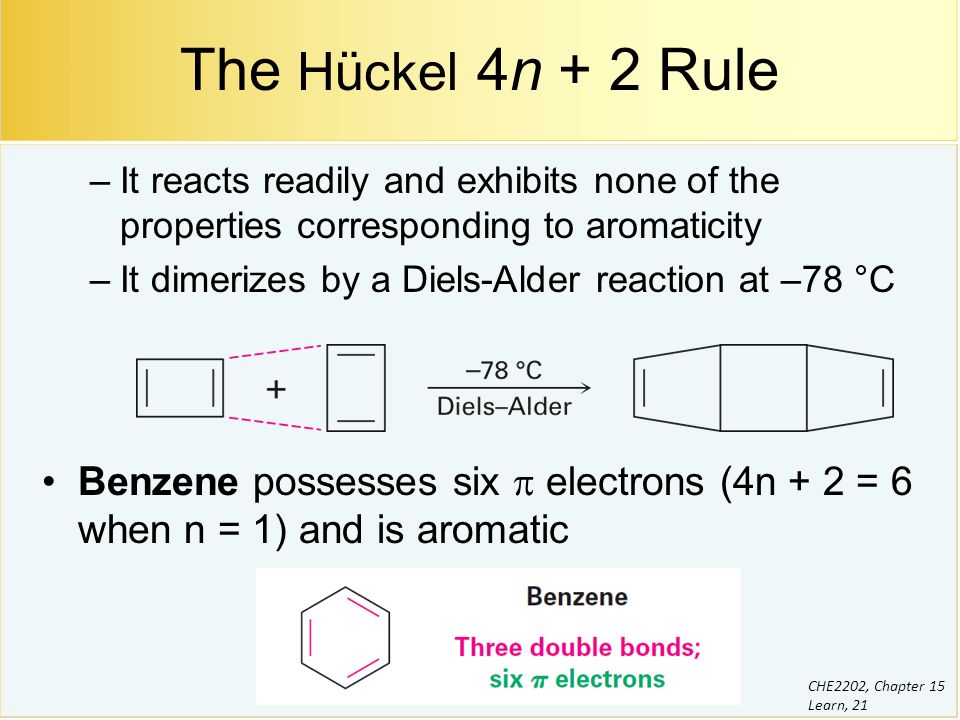 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
 What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic | 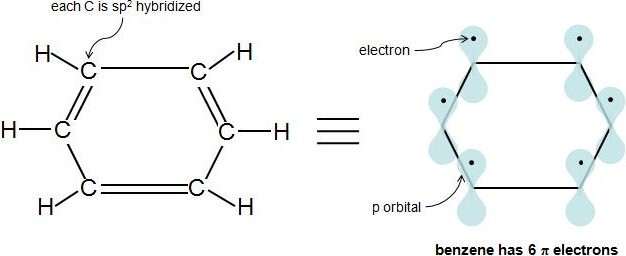.jpg?revision=1?readerView) What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic | 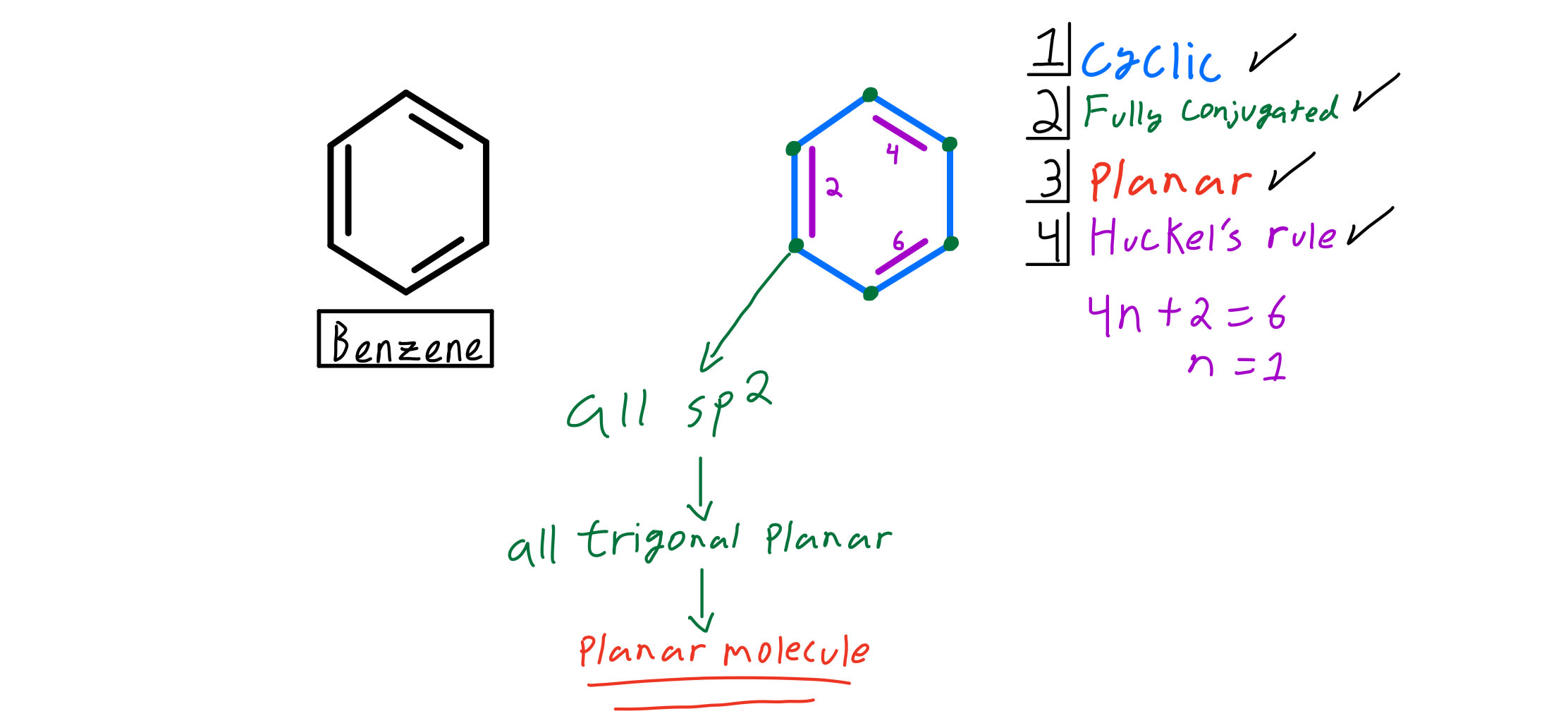 What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
 What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic | 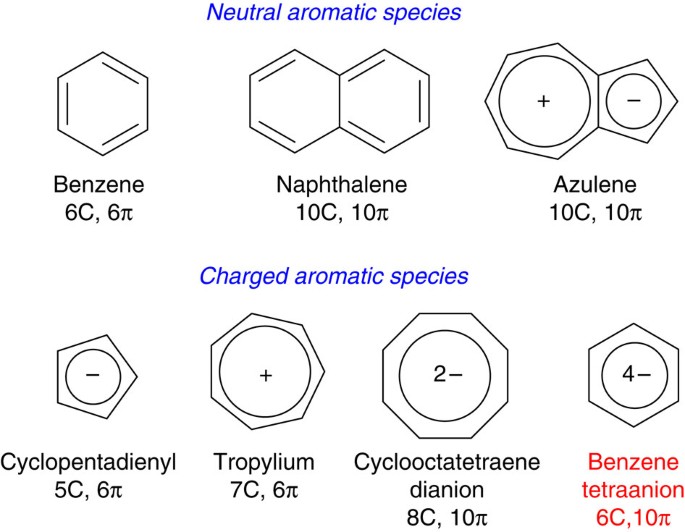 What Are Aromatic Compounds Socratic |
.jpg?revision=1) What Are Aromatic Compounds Socratic | 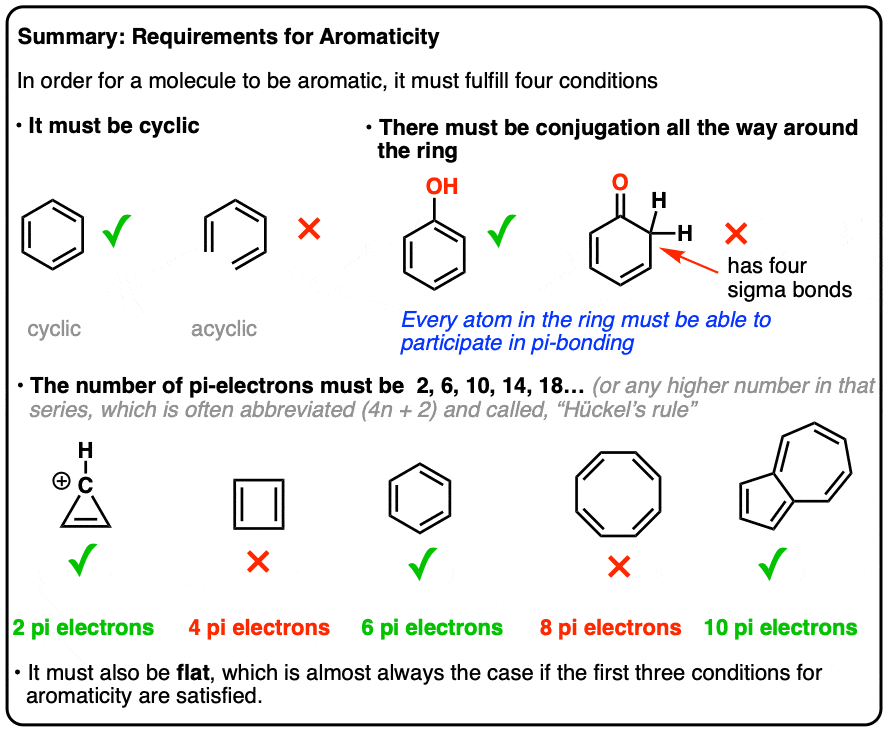 What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic | 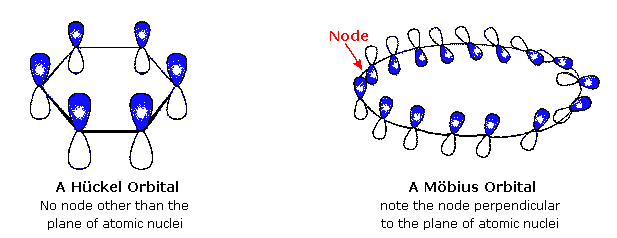 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic | 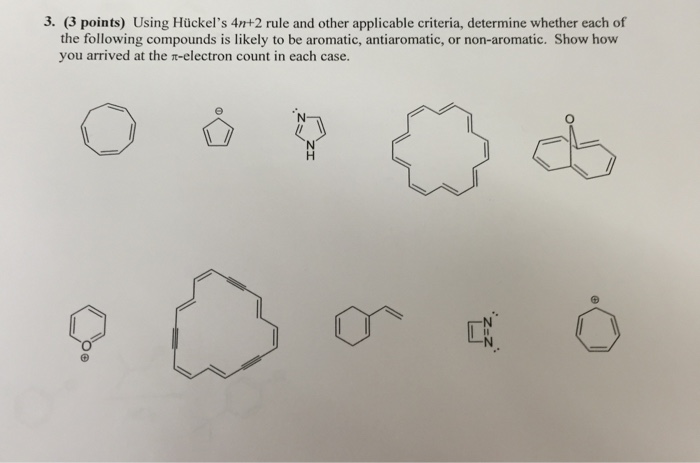 What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic | 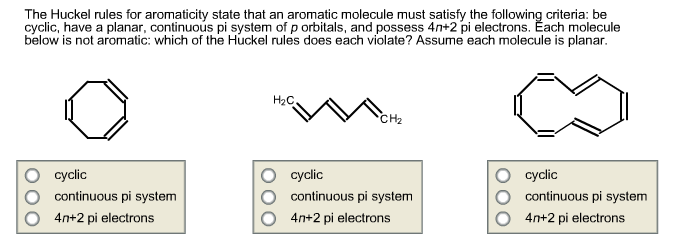 What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
 What Are Aromatic Compounds Socratic | 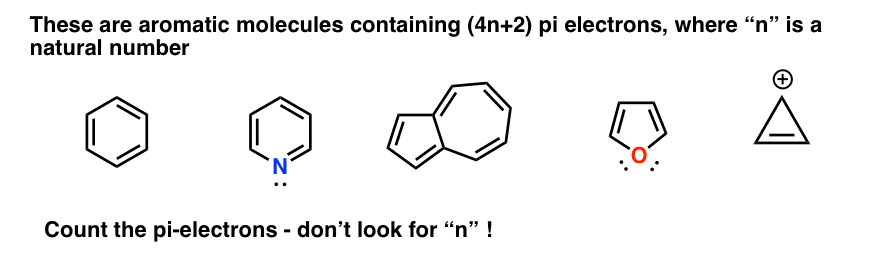 What Are Aromatic Compounds Socratic | 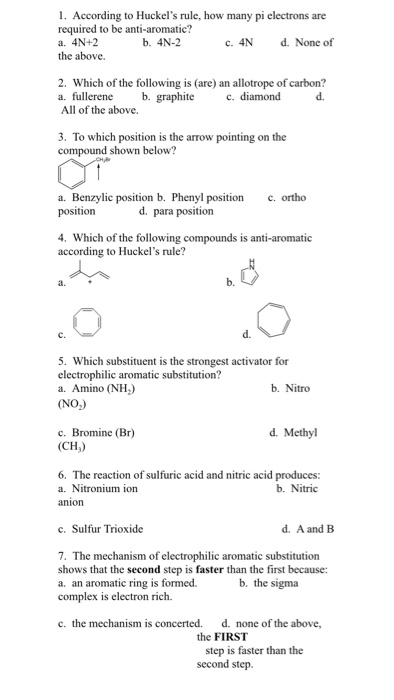 What Are Aromatic Compounds Socratic |
.jpg?revision=1&size=bestfit&width=440&height=181) What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic | 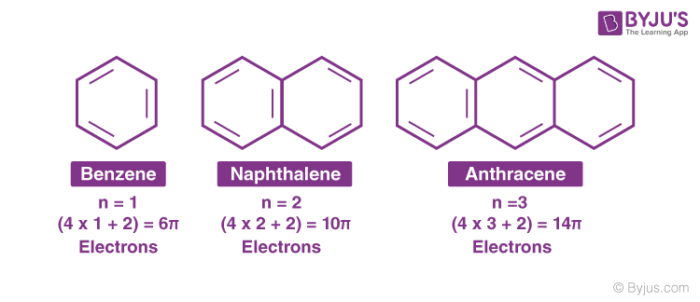 What Are Aromatic Compounds Socratic |
What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic | 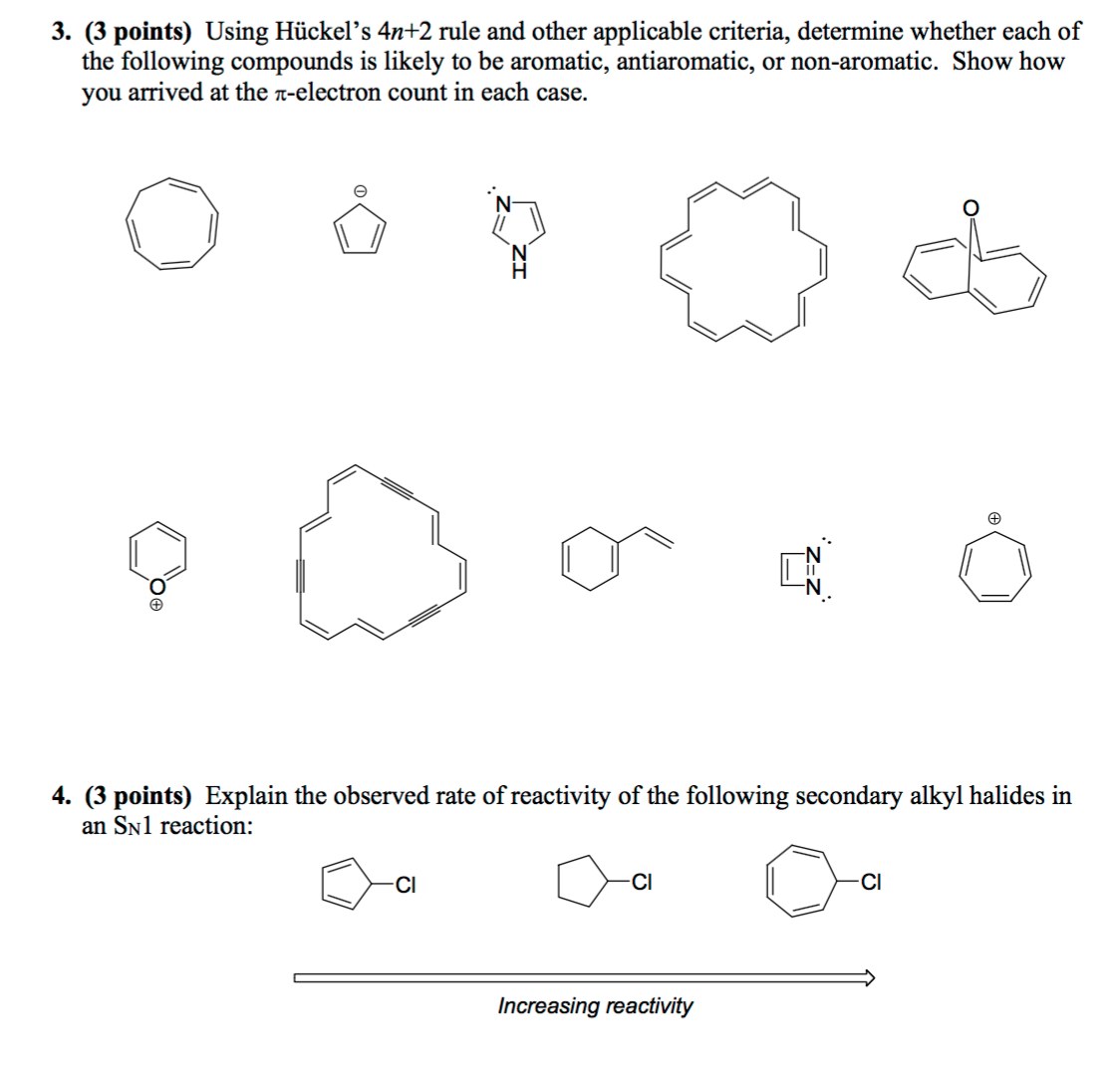 What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
 What Are Aromatic Compounds Socratic | 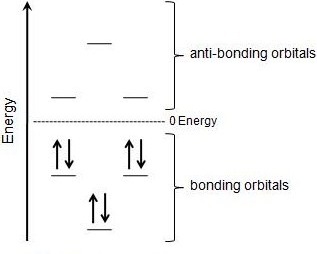.jpg?revision=1&size=bestfit&width=317&height=254) What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic | 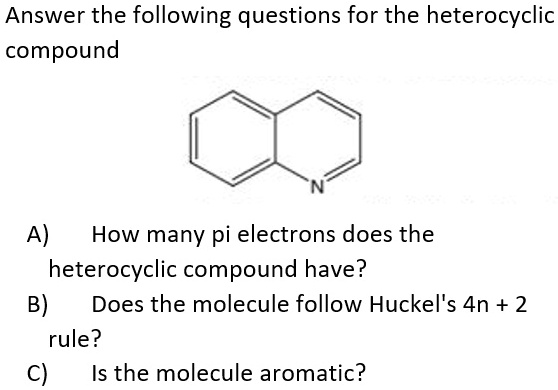 What Are Aromatic Compounds Socratic | What Are Aromatic Compounds Socratic |
「4n + 2 pi electron rule」の画像ギャラリー、詳細は各画像をクリックしてください。
 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
 What Are Aromatic Compounds Socratic |  What Are Aromatic Compounds Socratic |
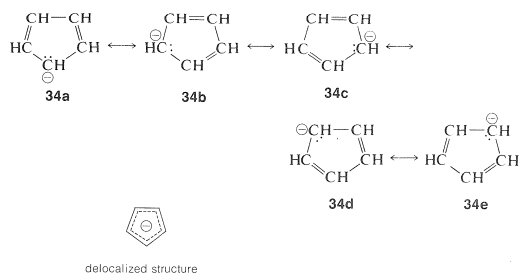
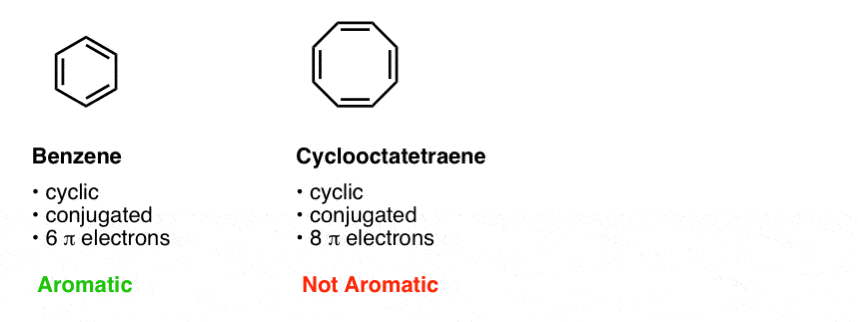
His rule states that if a cyclic, planar molecule has 4n2 π electrons, it is considered aromatic This rule would come to be known as Hückel's Rule What does 4n 2 mean in Benzene is an aromatic hydrocarbon because it obeys Hückel's rule Originally, benzene was considered aromatic because of its smell it has an "aromatic" odor It is now
Incoming Term: 4n + 2 pi electron rule,




0 件のコメント:
コメントを投稿